consists of a fixed combination of butalbital, acetaminophen, and caffeine. The role each component plays in the relief of the complex of symptoms known as tension headache is incompletely understood.
Butalbital is some kind of barbiturate, and barbiturates in general may appear in breast milk and readily cross the placental barrier. They are bound to plasma and tissue proteins to a varying degree and binding increases directly as a function of lipid solubility.
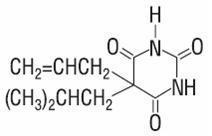 Elimination of butalbital is primarily via the kidney (59% to 88% of the dose) as unchanged drug or metabolites. The plasma half-life is about 35 hours. Urinary excretion products include parent drug (about 3.6% of the dose), 5-isobutyl-5-(2, 3-dihydroxypropyl) barbituric acid (about 24% of the dose), 5-allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid (about 4.8% of the dose), products with the barbituric acid ring hydrolyzed with excretion of urea (about 14% of the dose), as well as unidentified materials. Of the material excreted in the urine, 32% is conjugated.
Elimination of butalbital is primarily via the kidney (59% to 88% of the dose) as unchanged drug or metabolites. The plasma half-life is about 35 hours. Urinary excretion products include parent drug (about 3.6% of the dose), 5-isobutyl-5-(2, 3-dihydroxypropyl) barbituric acid (about 24% of the dose), 5-allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid (about 4.8% of the dose), products with the barbituric acid ring hydrolyzed with excretion of urea (about 14% of the dose), as well as unidentified materials. Of the material excreted in the urine, 32% is conjugated.
The in vitro plasma protein binding of butalbital is 45% over the concentration range of 0.5-20 mcg/mL. This falls within the range of plasma protein binding (20%-45%) reported with other barbiturates such as phenobarbital, pentobarbital, and secobarbital sodium. The plasma-to-blood concentration ratio was almost unity, indicating that there is no preferential distribution of butalbital into either plasma or blood cells.
According to safefetus.com Fioricet is a Class C drug. It says it does cross the placenta and can cause fetal abnormalities and brain tumors if taken while pregnant.
Butalbital is not suggested as a first-line treatment for headache because it impairs alertness, brings risk of dependence and addiction, and increases the risk that episodic headaches will become chronic.
Babies born to mothers who took medications containing butalbital while pregnant may exhibit withdrawal or addiction symptoms or breathing problems. Pregnant or nursing women should not take medications containing butalbital unless their doctor feels the benefits of the drug outweigh the risks. Taking butalbital while pregnant may result in miscarriage.
⚠️ Butalbital Use During Pregnancy: Risk of Miscarriage
Butalbital, a barbiturate component of drugs like Fioricet, is not considered safe during pregnancy, particularly in the first trimester. Its use has been associated with several risks, including:
🚨 Potential Risks of Butalbital During Pregnancy:
| Risk | Details |
|---|---|
| Miscarriage (Spontaneous abortion) | Some studies suggest a possible association, especially with first-trimester exposure. |
| Fetal CNS depression | Barbiturates cross the placenta and may depress fetal central nervous system activity. |
| Neonatal withdrawal syndrome | Chronic use may lead to physical dependence in the fetus, causing withdrawal symptoms after birth. |
| Congenital malformations | Limited and inconclusive evidence exists; barbiturates in general have been linked to birth defects in animal studies. |
📊 FDA Pregnancy Category (Historical):
- Butalbital: Category C
Animal studies have shown adverse effects on the fetus, and there are no adequate human studies, but the potential benefits may warrant use in pregnant women despite potential risks.
(Note: The FDA has replaced letter categories with a risk summary approach as of 2015.)
🧬 Why the Risk Exists:
- Butalbital affects GABAergic neurotransmission, which plays a key role in fetal brain development.
- It is lipophilic, allowing easy placental transfer.
- Risk increases with chronic or high-dose use, especially early in pregnancy.
✅ Recommendations:
| If Pregnant or Planning Pregnancy |
|---|
| Avoid butalbital-containing medications unless absolutely necessary. |
| Consult a healthcare provider for alternative headache or pain treatments. |
| If already using butalbital, discuss safe tapering to avoid withdrawal. |
| Use non-pharmacologic strategies for headache management when possible. |
